About
About Us
In Newcastle we have a number of Biobanks storing tissue for use in medical research.
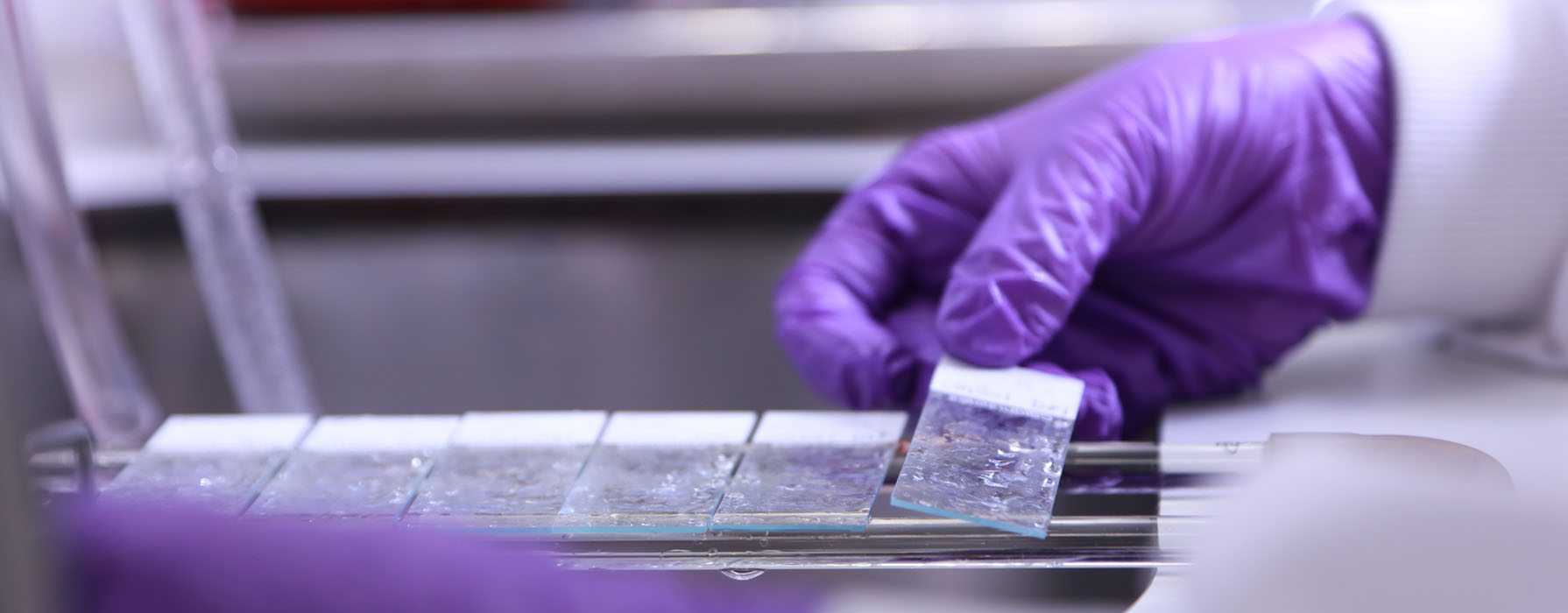
We store a wide range of samples, in disease areas such as:
- cancer
- mitochondrial disease
- neurodegenerative disease (e.g. Alzheimer’s)
- COVID-19
Patients or healthy volunteers generously donate these samples for researchers to better understand diseases.
The samples are vital to allow researchers to develop new diagnostic tests, drugs and surgical techniques.
We also offer a wide range of services and facilities to support researchers with their work.
Further information
Contact us for more information and to discuss your requirements.
Newcastle Biobanks is one of a number of facilities available for commercial use at Newcastle University. A full list of University Research Facilities can be found on the Services for Business website.
