|
|
Staff involved:John Bythell, Martin LeTissier, Tony O'Donnell, Mike Barer, Rory Cooney, Olga PantosFunding:NERC, Royal Society
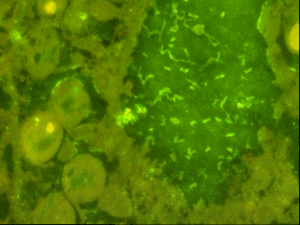
Fluorescent in situ hybridisation (FISH) using a eubacterial 16S probe (EUB338), showing brightly fluorescing bacterial cells in coral tissues (Acropora palmata) with white band disease. |
Comparative pathology of coral diseasesProjectThis new initiative (1999) uses a molecular approach to characterise the microbial community associated with a range of coral diseases, including both well-documented diseases that have been poorly described to date and a number of novel diseases that are undescribed or uncharacterised. Our approach involves the initial extraction of total DNA from disease samples and control tissues, followed by PCR amplification of ribosomal DNA using consensus 16S primers carrying a GC clamp (a 35-50 bp oligonucleotide rich in guanine and cytosine which stabilises the melting behaviour of DNA allowing PCR amplicons of different base compositions to be separated by DGGE (denaturing gradient gel electrophoresis). Following electrophoresis, separated bands are digitised, quantified and major shifts in the bacterial populations identified by the appearance or change in intensity of particular bands. Bands identified in this way are dissected from the gels, purified and re-amplified using PCR and sequenced. The sequence data is compared with those found in on-line databases (EMBL, RDP) to provide the closest relative if not the actual identity of the microorganisms involved. In parallel with this study, histopathological thin sections are also prepared using a novel agarose embedding technique which preserves both healthy and necrotic tissues in it's original configuration. Sections are probed for specific microbial groups using labelled oligonucleotide probes complementary to 16S ribosomal RNA and visualised by fluorescent labels (see figure). In this way it is possible to determine the microbial agents associated with specific phases of the disease. SignificanceIt is widely accepted that diseases of reef corals are an important factor in determining coral reef community structure, and that diseases may make a significant contribution to coral reef degradation seen throughout the Caribbean and elsewhere (Bythell & Sheppard 1993; Richmond 1993; Aronson & Precht 1997). Recent outbreaks of apparently novel diseases in reef corals have reached epidemic proportions in some regions and have generated considerable interest in the literature and media (http://coral.aoml.noaa.gov/). At least one study has raised the possibility that large-scale eutrophication and land run-off may have led to adaptive changes in soil-derived micro-organisms which have subsequently instigated coral disease (Smith et al. 1996; Nagelkerken et al. 1997). In another case, microbial infection (Vibrio AK-1) has been implicated in episodes of coral mortality due to bleaching (loss or damage to the algal symbionts) which are correlated with elevated temperatures (Kushmaro et al. 1997; Toren et al. 1998). Historically and ecologically the most important diseases in the Caribbean are White Band Disease (WBD) and Black Band Disease (BBD) (Garret & Ducklow 1975; Gladfelter 1982; Rützler & Santavy 1983; Peters 1993; Kuta & Richardson 1996). While these diseases are present at low levels in virtually all parts of the tropics, WBD has been the cause of an epidemic which has virtually eliminated what was previously the most common reef-building coral in large areas of the Caribbean. Coral reefs dominated by elkhorn coral Acropora palmata accounted for more than half of all reef types present in parts of the north-eastern Caribbean prior to the outbreak of the epidemic in the mid 1970s, but now represent less than 2% of reefs. The disease is still prevalent despite the dramatic reduction in the host populations. Although a major ecological phenomenon, very little is known about the aetiology and pathology of these diseases. It is clear that we must fully characterise the diseases and develop the tools necessary to identify the causal agents as a matter of urgency. Future directionsThe tools developed and sequence information on the microbial community will be invaluable in determining the causal agents of the diseases. The techniques we are using are largely free of the problems of culturability of the organisms involved. However, the next phase will require the culture of suspected pathogens to move on to test Koch's postulates and identify causal agents. References
|