Neuromuscular Junction Research Lab
About
Our facility was established in 2018 through a multi-user equipment grant from the Wellcome Trust.
The Applied Neuromuscular Junction Facility is a research facility for confocal microscopy and electrophysiological applications. The primary aim of the facility is to investigate the structure and function of neuromuscular junction (NMJ) using mouse models -mimicking human diseases- as well as human motor point biopsies.
The facility houses state-of-the-art imaging and electrophysiology equipment not only to allow researchers to investigate intracellular recordings of synaptic events from NMJs but also to capture high quality confocal images. In our facility you will have the opportunity to generate high resolution images of subcellular structures and molecular events with great sensitivity and high speed as well as detection of voltage recordings and ion channel currents in isolated nerve and muscle preparations.
Moreover, the Facility is equipped with state-of-the-art patch-clamp devices permitting high-resolution recording of the ionic currents through the plasma membrane.
We offer a range of services to meet your research needs, from the sample preparation, acquisition, analysis of multichannel fluorescence-based images and electrophysiology recordings to hands-on training in microscopy and electrophysiology.
Applications
Neuromuscular Junction (NMJ) Research
We use a combination of electrical recording and imaging techniques to analyse NMJ function on isolated nerve-muscle preparations, whether derived from patient biopsy samples or mouse muscles. Several muscles can be used for functional NMJ analysis representing those that are most suitable for electrophysiology (e.g. epitrochleoanoconeus) as well as others-most appropriate for the project -containing either predominantly fast-twitch (extensor digitorum longus) or slow-twitch (soleus) motor units. The required nerve and muscle preparations are dissected prior the experiments within the Facility.
Voltage recordings including two-electrode voltage clamp method is used to assess the properties of NMJ function and we collect the following data:
- Compound muscle action potential (cMAP)
- Miniature Endplate Potentials (mEPPs)
- Endplate Potentials (EPP)
- Miniature Endplate Currents (mEPCs)
- Endplate Currents (EPC)
Additional imaging methods to study NMF function:
- Synaptic vesicle dynamics
- Investigation of synaptic mitochondria and axonal mitochondrial trafficking
- Calcium dynamics
- Detailed NMJ morphology
Patch Clamp electrophysiology
We also provide patch clamp platform/optogenetics to measure membrane action potentials and ion channel currents as well as the activity of single ion channels from any isolated, genetically modified or differentiated cells or cellular tissues. Our instrumentation has the possibility to extend to external field potential recordings from MEAs (Multi Electrode Arrays) which would enable high throughput field potential recordings of brain slices, dissociate tissues, organoids, spheroids or 2-D neuronal culture.
These electrophysiology recordings are most commonly used to study the following:
- Measure the membrane properties of cells (e.g., resting membrane potential)
- Detect the presence of specific channels and receptors
- Study the function of membrane channels and receptors
- Measure the characteristics of action potential
- Detect synaptic transmission
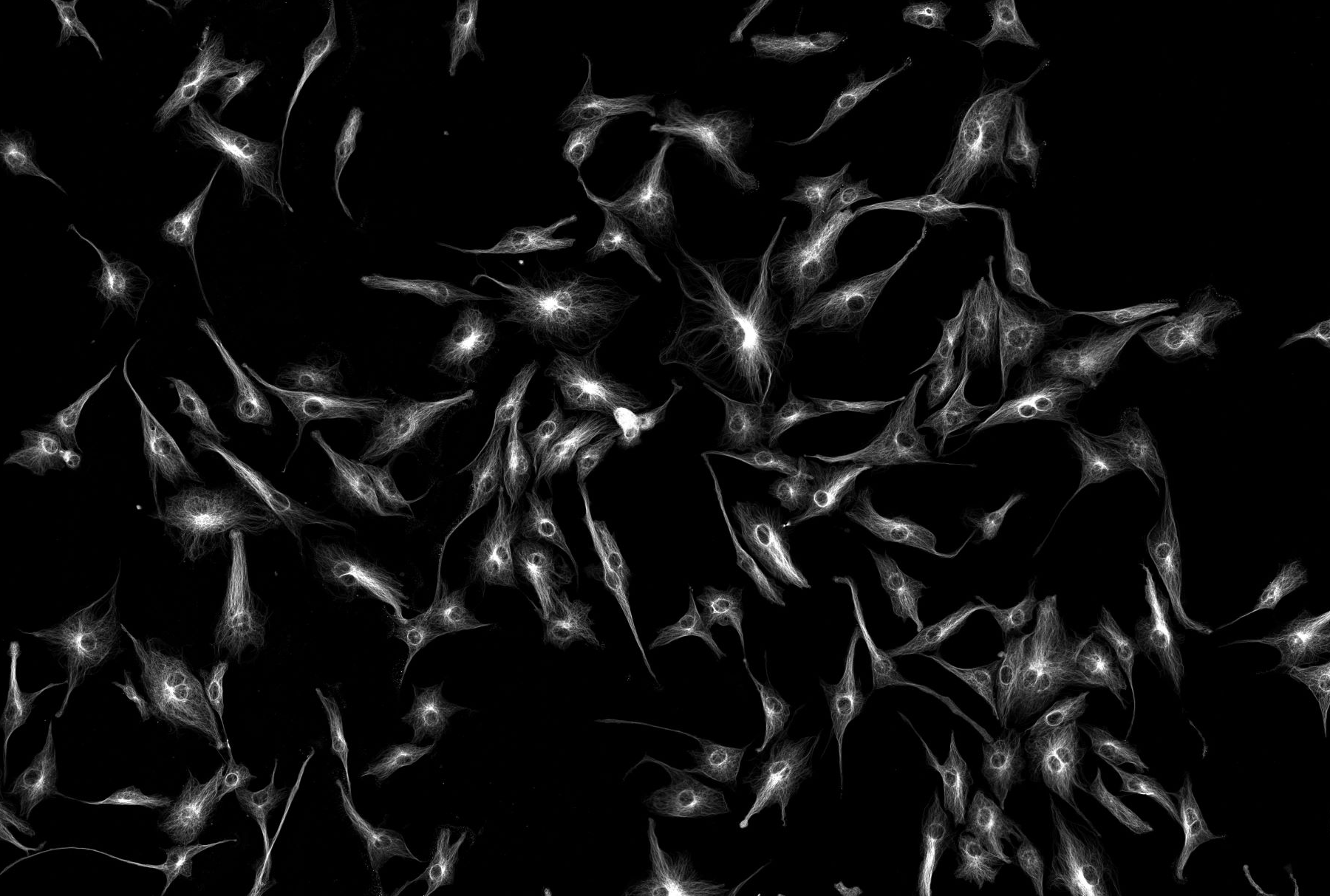
Confocal microscopy
This technology makes possible the acquisition of fluorescence-based images of cellular structures and molecular events from biological samples and nanomaterials. For example, cellular organelle structures and neuronal action potentials, as well as intracellular calcium oscillations and drug delivery of nanoparticles, can be visualized with high temporal and spatial resolution.
Imaging and Electrophysiology
The coupling of confocal microscopy with two electrode voltage-clamp or patch-clamp electrophysiology enables simultaneous acquisition of fluorescence images and bioelectrical signals from biological cells and tissues.
Instrument overview and booking
The following equipment is located in the NMJ Facility in the Medical School:
Stereo
Brightfield / Widefield Fluorescence
Confocal
Instrumentation
Here you'll find detailed information about the microscopes and electrophysiology rigs available at the NMJ facility:
Microscopes
Zeiss LSM880 Upright Confocal Microscope (built on an Examiner Z1 body)
- Semi-automated microscope with a stationary fixed stage
- Long working distance, water dipping objectives
- Equipped with two multi-Alkali PMTs and 32-channel spectral GaAsP PMTs (up to 13 fps at 512 × 512 pixels)
- Airyscan detector
- Capabilities:
- Up to 1.7x better resolution (in all three dimensions)
- Improved sensitivity with 4-8x higher SNR
- More gentle excitation (less photobleaching and phototoxicity)
- Spectral unmixing capabilities:
- Acquire 5-8 dyes in parallel with spectral imaging
- Linear unmixing post-acquisition segregates mixed fluorescence signals from dyes and cellular autofluorescence
- Laser lines: 355, Argon (tuned to 458,488,514), 561, and 2 HeNes (594 and 633).
- Filter sets: DAPI/FITC/RFP
- Zeiss Colibri 7 multi-LED illumination system (630/590/555/511/475/430/385)
- Hamamatsu ORCA-Flash-4.0 V3 sCMOS camera for widefield imaging (speed up to 100 fps)
- The system runs on Zen black software package
- Experiment designer for advanced acquisitions
Zeiss Examiner Z1 Wide-field Fluorescence microscope
- Semi-automated microscope with a stationary fixed stage
- Long working distance, water dipping objectives
- Zeiss Colibri 7 multi-LED illumination system (630/590/555/511/475/430/385)
- Hamamatsu ORCA-Flash-4.0 V3 sCMOS camera for widefield imaging (speed up to 100 fps)
- The system runs on Zen blue software package
- Experiment designer for advanced acquisitions
Workstation computer (For Analysis) is available within the facility.
The facility analysis PC is available to use for image and data analysis with updated Fiji and Zeiss confocal software allowing remote desktop access for Huygens and Imaris. This is available to use free of charge for microscopy user account holders.
Electrophysiology rigs
Current and 2-electrode voltage clamp recording Rig (for nerve and muscle preparations)
Coupled with both Zeiss LSM880 and Zeiss examiner Z1 microscope
- Axon Axoclamp 900A Microelectrode Amplifier
- Axon Digidata 1550B digitizer
- Digitimer Constant voltage stimulator#DS2A-Mk.II
- 2 x Scientifica PatchStar Motorised Micromanipulator (LBM7) with control cube
- Warner Instruments SH-27B Solution In-Line Heater 64-0102
- Large Bath Chamber platform-RC-27L
- Axon pCLAMP 10 software
- Digitimer Humbug noise eliminator
- Perfusion system
- Carl Zeiss SVB-1 Microscope Signal Distribution Box
- Carl Zeiss Interface box
- Anti-vibration table + Faraday cage
Patch clamp, voltage clamp and current clamp recording Rig (in preparation)
Coupled with Zeiss LSM880 microscope
- Axon Axopatch 200B Microelectrode Amplifier
- Axon Digidata 1550B digitizer
- Digitimer Constant voltage stimulator#DS2A-Mk.II
- 2 x Scientifica PatchStar Motorised Micromanipulator (LBM7) with control cube
- Warner Instruments SH-27B Solution In-Line Heater 64-0102
- Hamilton electronic multi-line perfusion system
- Ultra quiet large Bath Chamber platform-RC-27LD
- Axon pCLAMP 10 software
- Digitimer Humbug noise eliminator
- Carl Zeiss SVB-1 Microscope Signal Distribution Box
- Zeiss Colibri’ trigger box for optogenetic stimulation (7 colors)
- Carl Zeiss Interface box
- Anti-vibration table + Faraday cage
Training and support
The NMJ Facility provide advice on project planning, sample preparation and the most appropriate instrumentation to use for a given application. Contact us to discuss your research requirement and request an account and/or training through the PPMS website.
The Facility also offers training opportunities for graduate students, postdoctoral fellows, young investigators and staff on imaging and electrophysiology techniques. We provide comprehensive training in:
- sample preparation
- microscopy
- electrophysiology
- data and image analysis.
Researchers may be trained for unassisted use of all core instrumentation, while full-service assistance by facility staff is also available for qualitative or quantitative image capture, electrophysiological data collection and data analysis.