Microscopic origin of chirality
Nature’s mirror – the code for chirality
Published on: 8 February 2016
How information is transferred from biological molecules to crystalline surfaces could pave the way for the development of new drugs.
New research, published today in Nature Chemistry, explains how biological molecules can change the shape of minerals by controlling how they link together.
Investigating why crystals of achiral minerals obtain a chiral shape – that is, how they take on a left- or right-handed nature – the research team showed how metal surfaces could be re-shaped by chiral molecules.
The importance of chirality – or ‘handedness’ – in drug development was brought to light in a devastating way almost half a century ago with the development of the Thalidomide drug.
Prescribed widely to pregnant women for the treatment of morning sickness, it was later discovered that Thalidomide is a chiral molecule and while the left-handed molecule was effective, the right-handed one was highly toxic. As a result, thousands of children around the world were born with severe birth defects.
Paving the way for new drugs
Werner Hofer, Professor of Chemical Physics at Newcastle University, UK, and one of the authors on the paper, says this new research furthers our understanding how chiral molecules behave and could pave the way for the development of new drugs and other synthetic materials.
“In the biological world, we see inorganic minerals being shaped with remarkable control but until now we haven’t understood how it was happening at the level of the atoms,” explains Professor Hofer.
“Now we see that the organic molecules are acting as a scaffold, dictating where the minerals are placed and how they are linked together - a bit like building blocks. And as they do this, the biomolecules transfer their left or right-handed nature, or chirality, to the crystal structure.
“By understanding this process, we can now force materials to behave in a certain way, using biological plans to create the shapes and structures that we want. This has huge potential in the fields of drug synthesis and microelectronics.”
Professor Roman Fasel, who led the study and is based at Empa, the Swiss Federal Laboratories for Materials Science and Technology, adds:
“Single-handed metal surfaces are of considerable interest in enantioselective heterogeneous catalysis - a chemical strategy to produce single-handed molecules in a selective way.
“Our work reveals an easy way to obtain such surfaces, simply by adsorbing a specific single-handed molecule that re-shapes the metal into the desired chiral morphology.
“However, it must be noted that the present results only provide a proof-of-principle – to put this into practice, the challenge will be to identify the “good” molecule that creates the specific metal surface morphology suitable for the desired catalytic reaction. That is not an easy task by any means, but we hope that our work may stimulate efforts along these lines.”

Chirality in nature
Chirality – or ‘handedness’ - is a striking property of the biological world. Many organic molecules, including glucose and most biological amino acids are chiral and the DNA double helix in its standard form always twists like a right-handed screw.
Chirality can also be seen in organisms. Snails, for example, can show chirality or ‘handedness’ — some individuals have shells that spiral in a right-handed direction, others have left-handed shells.
“It means that they cannot be superimposed onto their mirror-image,” explains Professor Hofer, a chemical physicist at Newcastle University, UK.
“Your left and right hand are chiral - mirror images of each other but no matter how you put them together you can’t exactly superimpose one onto the other.
“But while this phenomenon is common in the biological world, it’s rarely seen in mineral structures, except in those that have been biologically formed and a lot can be learned about how they are created by studying how chiral information is transferred from molecules to crystalline surfaces.”
In this latest study, the international research team - involving experts from the UK, Switzerland, Hungary Italy, China and the US - explain how handedness is transmitted and what the underlying mechanism is at the atomic scale.
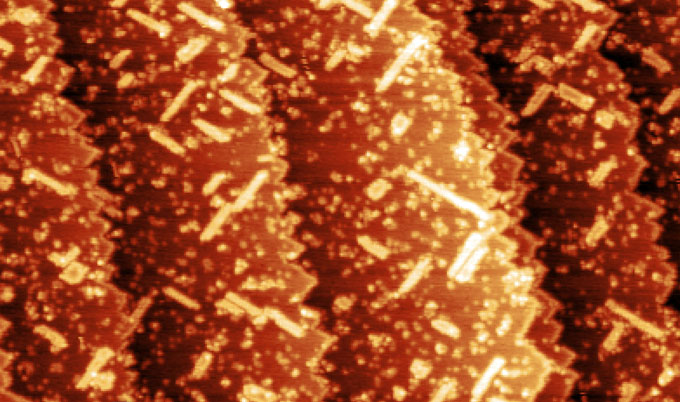
Using the organic molecule hemifullerene imprinted onto a copper surface, the team saw how the copper surface atoms were re-arranged into a chiral pattern due to the formation of chemical bonds.
“We started with a very ordered, achiral surface and what we ended up with was a classic chiral pattern,” explains Professor Hofer.
“The organic hydrocarbon had restructured the metal surface in such a way that chirality had become imprinted onto the metal.”
Chirality and drug development - the Thalidomide tragedy
Thalidomide was prescribed widely to pregnant women between 1957 and 1962 for its benefits in reducing morning sickness.
However, when taken during the first trimester of pregnancy, Thalidomide prevented the proper growth of the foetus and the result was that thousands of children around the world were born with severe birth defects.
Thalidomide is a chiral molecule and the drug that was marketed was a 50/50 mixture of left and right-handed molecules. While the left-handed molecule was effective, the right-handed one was highly toxic.
The essential oil carvone is another (of many) examples of where the right and left handed forms of the molecule are polar opposites – one smelling like caraway, the opposite handed isomer like spearmint.
“The Thalidomide tragedy highlights the important role played by chirality in biological systems and what happens when we get it wrong,” says Professor Hofer.
“This research will hopefully further our understanding of this process and help us to accurately synthesise new drugs and materials in the future.”
Reference: Microscopic origin of chiral shape induction in achiral crystals. Wende Xiao, Karl-Heinz Ernst, Krisztian Palotas, Yuyang Zhang, Emilie Bruyer, Lingquing Peng, Thomas Greber, Werner Hofer, Lawrence Scott and Roman Fasel. Nature Chemistry 2016
DOI: 10.1038/NCHEM.2449