proofreading errors cause inherited blindness
Widespread errors in “proofreading” cause inherited blindness
Published on: 12 October 2018
Mistakes in “proofreading” the genetic code of retinal cells is the origin of a form of inherited blindness, retinitis pigmentosa (RP) caused by mutations in splicing factors, research has revealed.
This new understanding of the disease process, published today in Nature Communications, is leading to the development of a gene therapy for RP caused by splicing factor defects.
The work, led by Professor Majlinda Lako at Newcastle University, investigated how a common form of inherited blindness, retinitis pigmentosa, is caused by genetic defects in splicing factors.
Splicing factors are important protein components of the gene proofreading or “splicing” mechanism that is found in all cells. Some sections of our DNA, known as introns, are removed or spliced out by the cell during protein construction, so that only the final intelligible genetic code remains. This is because the introns do not actually provide any meaningful instructions for making proteins. Variations in splicing can cause very different consequences on the formation or function of cells, including retinal cells.
The scientists were able to create a “retina in a dish” using stem cells derived from the skin samples donated by retinitis pigmentosa patients at the University of Leeds.
This cell model enabled the team to compare retinal cells to others in the body. These cells are normally very hard to obtain as they would previously have had to be donated from the retina, usually after death.
Using this model, the researchers have shown that defects in splicing factors cause defective proofreading of components of the editing machinery itself. This counter-intuitive effect results in a “vicious cycle” of disruptive misinterpretation of the genetic code. The formation and functions of a special type of retinal cells, retinal pigment epithelial (RPE) cells, are the most severely affected. These cells are essential for supporting and nourishing photoreceptors (rod and cone cells), so when they go wrong the light-processing function of the retina breaks down, resulting in sight loss.
The study shows, for the first time, how genetic defects in splicing factors cause variations in the proofreading of retinal genes, leading to defects in retinal cell function and their eventual degeneration in retinitis pigmentosa.

Potential pathway to future treatments
Professor Lako’s team went on to show that CRISPR-Cas9 gene editing could be used to correct the genetic defects in a particular splicing factor. This also corrected the function of the RPE and rod and cone cells in their laboratory model, indicating a potential pathway to future treatments.
Professor Lako said: “This research gives us much deeper and broader insights into how splicing factors cause retinitis pigmentosa, enabling the next step in our research - the design of gene therapies for future treatments.”
Co-author Professor Colin A. Johnson, from the University of Leeds, said: “We've been puzzled by the genetics behind these unique forms of inherited blindness for over 20 years. Our study is the first to really make sense of how these conditions develop, and I'm now very hopeful that this will lead to clinical trials for new treatments within five years.”
Tina Houlihan, Chief Executive, RP Fighting Blindness said: “We are delighted that our support has enabled the group to publish these important findings in Nature Communications. We look forward to seeing the development of this work through our newly awarded grant, which will allow Professor Lako and the team to further understand the mechanisms underlying this type of RP and progress towards the targeted treatment strategies our community needs.”
Professor Mike Cheetham from UCL, scientific advisor for RP Fighting Blindness commented: “It has been a conundrum why genetic changes in ubiquitous and highly conserved ‘splicing factors’ cause RP. This exciting work is a major step towards understanding how these changes in splicing factors lead to RP, and was only possible by using stem cells made from affected individuals and turning them into a ‘retina in a dish’. There is still much to learn about why splicing factors are so necessary for the retina to function and how we might repair, or treat, this in individuals with this type of RP, but this work will focus research on the right models and pathways for future development.”
The research was led by four teams in collaboration with 38 researchers worldwide: Prof. Majlinda Lako (Newcastle University), Prof. Colin A. Johnson (University of Leeds), Dr. Sushma Nagaraja-Grellscheid (University of Durham) and Prof. Reinhard Luehrmann and Dr. Sina Mozaffari-Jovin at the Max Planck Institute in Gottingen, Germany.
RP with splicing factor defects affects around 23,000 people in the UK and up to 2.5 million worldwide.
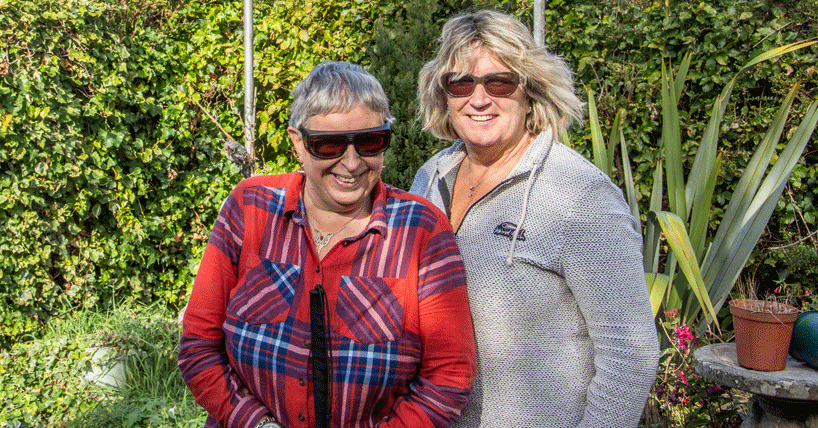
Living with RP – the patients’ story
Sisters Jane and Helen Walker from Leeds both have retinitis pigmentosa and donated skin cells to the University of Leeds, which allowed the researchers to make this genetic breakthrough.
Jane, 55, a retired school meals assistant, said: “We always used to struggle at school. It was hard to read and we couldn’t even see the blackboard, so we used to just muddle through. Mum and Dad only told us when we’d left school that we were visually impaired, they just wanted us to have a normal life as best we could.
“Mr McKibbin at St James’s Hospital got us involved in the research. I wanted to see if this study might help anyone else that shares our pain. It’s wonderful news that this research might soon be able to help other people.”
Helen, 54, trains care workers and consults for a care home. She said: “I’ve got a tiny, tiny tunnel of vision now – it’s like looking through a pinhole. Having no peripheral vision at all is really frightening. Anything on our periphery we just don’t see - it might be centimetres from our central vision.
“I became independent by using a guide dog called Tasmin. Tasmin’s a life-saver but she’s coming up to retirement age, and I’ve become very reliant on her. Believe me trying to navigate and negotiate streets, furniture, curbs, and particularly cars parked on pavements, is incredibly difficult with just a long white cane.
“This research came at the right time and I was more than happy to do it. You’ve always got to have hope. You do it for other people, but there’s also a selfishness as well, because I want to know what it would be like to have more sight.”
REFERENCE: Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nature Communications. Doi:10.1038/s41467-018-06448-y