Conversation: Antibiotic resistance
Comment: Antibiotic resistant ‘superbug’ genes found in the Arctic
Published on: 29 January 2019
Writing for The Conversation, Professor David Graham discusses the rapid spread of antibiotic resistance and the threat it poses.

Antibiotic resistance in bacteria is spreading rapidly worldwide and has even been called a global threat to humans as serious as climate change. Excessive and imprudent use of antibiotics is usually blamed, but the rate and extent of the spread can’t be explained by overuse alone.
Antibiotic resistance has now been found in remote parts of the world where humans and antibiotics are scarce or absent. Recently we published a study in Environment International that reported antibiotic resistant genes in Svalbard in the High Arctic.
Antibiotic resistance itself is natural, but continued human exposure to antibiotics has selected for progressively stronger bacteria. These bacteria often acquire antibiotic resistance genes that allow them to make powerful defence proteins. As more antibiotics are used new forms of resistance evolve, including multiple drug resistance in pathogens, creating a health crisis.
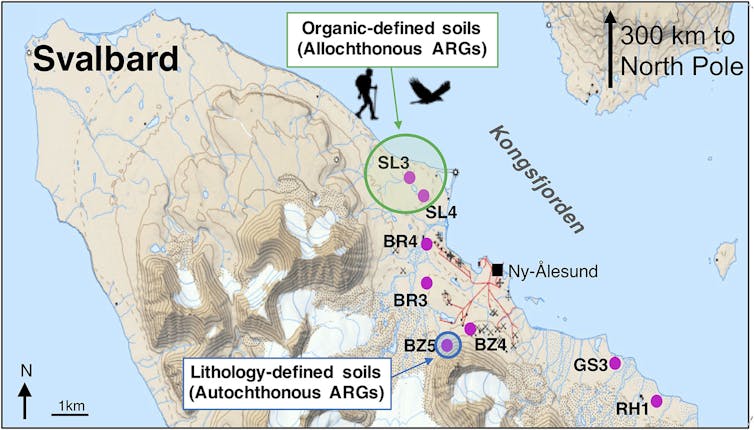
For our research, we extracted bacterial DNA from 40 soil cores at eight locations along Kongsfjorden on the west coast of Spitsbergen on the Arctic Ocean, 300km from the North Pole. This location is fairly unusual in the Arctic for its vibrant wildlife populations and neighbouring fjord which does not freeze. We screened the Arctic soil DNA and quantified 131 antibiotic resistance genes associated with nine major antibiotic classes. These include aminoglycosides, carbapenem, macrolides and β-lactams – many of which are used to treat human and veterinary infections.
Some of the detected genes, some of which are almost certainly not “local” to the Arctic, can confer resistance to multiple drugs. These “foreign” genes were found in highest concentrations near fresh water sources – areas where wildlife tend to congregate.
One antibiotic resistance gene we found is called “blaNDM-1”. This gene confers resistance in bacteria to carbapenem antibiotics, one of our last resort remedies to infectious disease. We found this gene in soils near a small Arctic lake in 2013, but it was first detected in a hospital patient in India in 2007, and was later found in surface waters in urban India in 2010.
Although not explicitly from India, BlaNDM-1 was first identified on one side of the world and migrated to the other in less than three years, ending up in a place where few humans reside and where antibiotics are scarce. How did it get there?

A migrating resistance
Places with inadequate sanitation often have higher levels of resistance genes and bacteria in the surrounding water and soil. Local overpopulation, poorly controlled antibiotic use and incomplete wastewater treatment means resistance is more readily selected in these places.
Local water and food supplies become contaminated with faecal matter, providing a pathway for resistance genes and their bacterial hosts to be ingested by humans and wildlife – hitching a ride from one region of the world to another. It’s unclear whether it’s a single migration via a bird or a human, or a chain reaction of exposures. By whatever pathway, resistance genes are moving fast and to places where antibiotics are not present.
As antibiotic resistance migrates, it also changes as it passes through animals and the wider environment. This complicates tracing these genes from their origins to where they end up. In the case of blaNDM-1, it was initially detected as a single gene in a few places, but there are now numerous variants which have been found in tens of countries, now including the Arctic.
If resistance genes migrate via humans or wildlife to other locations, especially places with inadequate local sanitation, such genes and bacterial hosts might be selected in subsequent human and wildlife populations. This is what we think is happening worldwide. These genes move around the world with people and other animals, seeding new places with resistance potential.
Reducing antibiotic use is critical to tackling bacterial resistance. In the UK, this is outlined in the five-year action plan announced by the health secretary, Matt Hancock. However, reducing local antibiotic use may have a limited effect on global resistance unless environmental pathways are not given greater consideration. Improving sanitation and water quality worldwide must be part of the fight against antibiotic resistance.
There is one positive note from our High Arctic study, however. Although we detected genes like blaNDM-1, we also found other interesting and unexpected antibiotic resistance genes. We found one gene that codes for multiple drug resistance in Tuberculosis bacteria in almost all of our soil cores. This gene was not explicitly related to faecal matter, which means it is almost certainly natural to this remote environment.
Read more: It’s the age of the antibiotic revolution, not apocalypse
This might sound like bad news, but it tells us where this specific resistance gene might have originated, and understanding more about this gene could help us combat resistant tuberculosis in future. This also suggests that information from remote soils, like those in Svalbard, might provide a valuable source of undiscovered drugs.
That said, focusing efforts on developing new arsenals of antibiotic drugs may not be enough. It additionally would be wise for wealthier countries to help poorer ones improve water quality and sanitation, even if it is only providing toilets to reduce open defecation. Smarter use of antibiotics in agriculture and medicine is a necessary step for tackling resistance, but only a comprehensive approach will reduce the evolution and spread of antibiotic resistance.
David W Graham, Professor of Ecosystems Engineering, Newcastle University
This article is republished from The Conversation under a Creative Commons license. Read the original article.



