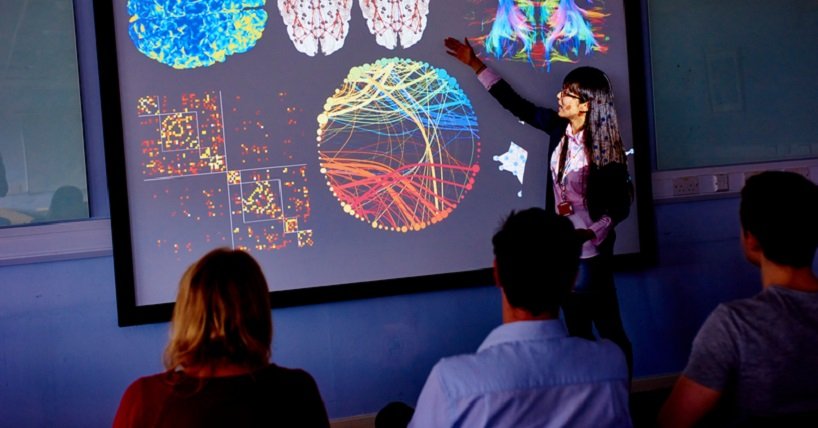
Regenerative Medicine, Stem Cells and Transplantation
This theme brings together a team of internationally-recognised scientists.
Our unifying goals are innovation, validation and implementation of novel regenerative medicine therapies. Transforming clinical outcomes across the spectrum of chronic disease.
Our interdisciplinary research programmes explore the biological mechanisms of tissue injury and repair in health and disease. Our mechanistic and clinical research benefits from powerful human stem cell and primary tissue models.
Our objectives are to:
- provide biological insights into organ development and maintenance of functional mass throughout life
- provide understanding of the molecular and cellular mechanisms involved in tissue repair
- understand the role of immune system in tissue repair and regeneration
- discover new diagnostic and prognostic biomarkers of tissue damage and repair. Both in endogenous disease and following therapeutic intervention. Including gene therapy, cell, tissue and organ transplantation
- pioneer innovative regenerative medicine therapies. Including gene therapy, stem cell transplantation and organ replacement therapy
- provide a collaborative, multi-disciplinary environment that promotes recruitment and training of early career scientists
- broadening the impact of our research to improve life quality and longevity. Engaging with key stake-holders: public, patients, schools, hospitals and industry
Research Infrastructure
Newcastle has a strong reputation in this area and boasts an impressive infrastructure. Regenerative Medicine is a key strand for Newcastle Health Research Partnership, our regional Academic Health Science Centre.
We work alongside The Institute of Transplantation at the Freeman Hospital, the only UK centre providing all solid organ transplants, as well as pancreatic islet cell transplantation a programme enabled by our research.
We are a regional centre for the MRC Quality in Organ Donation (QUOD) initiative. This unique resource aims to enhance transplant outcomes. Speed up translation of advanced therapy organ replacement and enable endogenous organ regeneration.
The Innovate UK Northern Alliance Advanced Therapies Treatment Centre is co-led by Newcastle. It fosters advanced therapy trials and clinical adoption locally, regionally and nationally.
The Transplant Regenerative Medicine Labs provides a research hub for donor organ perfusion. A platform for maintenance and resuscitation before transplantation. It develops processes for ex vivo delivery of advanced therapeutics to cells, organoids and whole organs. Newcastle Advanced Therapies provides bespoke GMP clean room manufacturing facilities. These include gene therapy medicines, somatic-cell therapies and tissue-engineered products.
Research Impact
Newcastle is a leading centre for wide-ranging advanced therapies innovation and clinical trials. Including gene therapy for neuromuscular disease. Inherited and age related retinal disease and haemophilia. We are a leader in Haematopoietic Stem Cell innovation and transplantation.
We pioneered transformative limbal stem cell therapy for painful sight loss. We translated Tolerogenic dendritic cell therapy for rheumatoid arthritis from bench to bedside. Expertise and experience in CAR-T cell therapy enabled the NHS funded regional CAR-T service. Pluripotent stem cell derivation and modelling has provided a platform from patient tissue to generation of functional corrected cells for transplantation
Latest Research News
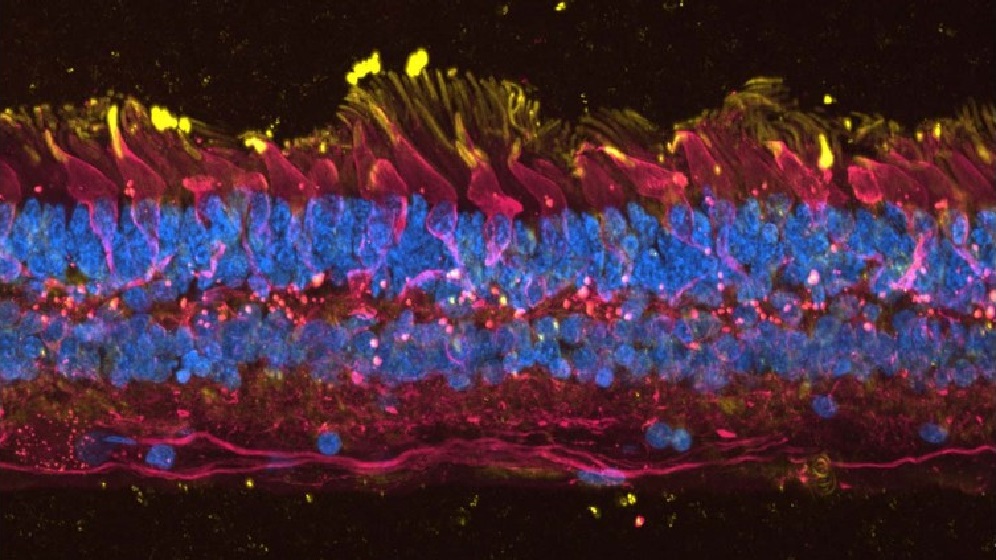
Eye Stem Cell Research
Our aim is to understand the development of the human eye during normal development and in blinding disorders. Such as Age-Related Macular Degeneration, Retinitis Pigmentosa and Limbal Stem Cell Deficiency.
We are committed to improving the lives of patients and developing cellular therapies for clinical trials. Our expertise in corneal epithelial stem cell biology, has led to phase 1/2 clinical trial for Limbal Stem Cell Deficiency. To date this has restored vision and quality of life in 34 patients.
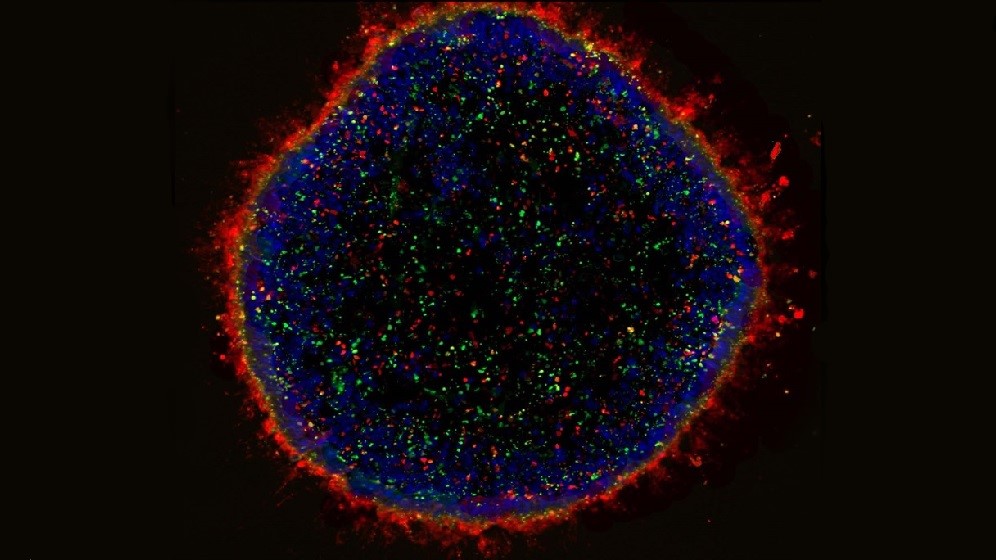
We use induced pluripotent stem cells (iPSC) to model eye diseases in the laboratory. From this we can understand the pathological mechanisms and screen potential therapies. We are part of an international consortium performing clinical trials of iPSC-derived RPE transplants in patients with Stargard’s disease.
Our group has pioneered the generation of lab made mini retinas. The aim is to use these in clinical trials to replace damaged retinal cells.
Find out more on our lab website.
Contacts
Tissue Engineering – 3D Bioprinting
We aim to engineer functional replacements and temporary “bridge” tissues using a modular approach. Developing model systems to study physiological and pathophysiological corneal tissue formation.
We focus on the application of innovative biomaterials to regenerative medicine. In particular, the application of functionalized peptide/polymer water-based gels. Exploring ways they could improve stem cell bioprocessing, storage (shipment) and subsequent engraftment.
These gels display self-assembling properties. We aim to exploit this to create intelligent coatings that control the spatiotemporal positioning of stem cells. Producing bioprosthetic tissues for tissue replacement or wound healing therapies.
Tissue engineering for transplantation is a key area of expertise. Another is the conception of functional cell-based biological constructs for a specific biotechnological need. We have denoted this process as ‘super tissue engineering’. The rational design of cell-based constructs that have limited but exceptional biotechnological function.
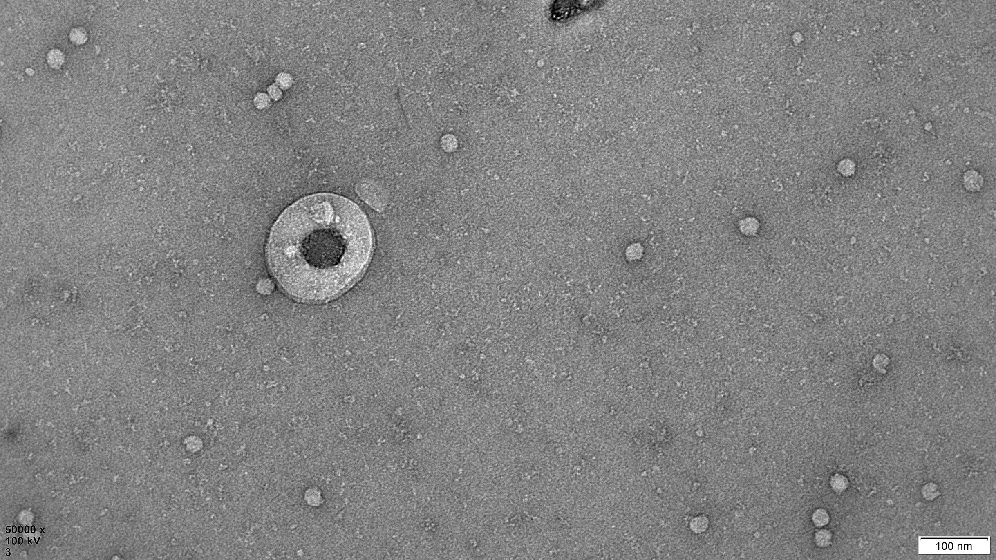
Human Extracellular Vesicle Research
We are evaluating the therapeutic potential of extracellular vesicles (EVs) secreted by cells. Currently used as cell therapies for tissue repair/regeneration and graft-versus-host disease. Mainly mesenchymal stem/stromal cells (MSC) and regulatory T cells (Treg).
EVs are small membranous particles carrying signalling molecules to their parent cells. By delivering these molecules to target cells they help achieve the therapeutic benefits. Our research sheds new light on how these therapeutic cells work. It evaluates the potential of using EVs as a cell-free therapy. Or in combination with their parent cells to improve the cell therapy.
We are part of the multidisciplinary Tissue Engineering and Regenerative Therapies Centre. We investigate the role of MSC-EVs in immunomodulation and joint tissue regeneration. This will aid identifying treatments for early stage osteoarthritis.
Contact
- Xiao Wang
Cardiac Regeneration and Tumour Formation
Adult tissues retain stem cells that are capable of multilineage differentiation. We focus on fundamental regulatory mechanisms that restrict this differentiation during normal development. As well as the cellular proliferation, differentiation and plasticity of these cells in response to injury or disease.
We have identified a population of stem cells located in the heart and bone marrow. These cells are known as side population (SP) cells. They are capable of migration into injured myocardium and differentiation towards cardiac lineage.
We investigate the presence of cancer stem cell like cells in cases of recurrent cancers such as breast and ovarian cancers. We have identified cancer stem cell populations in breast cancers. As well as the presence of SP cells in both tissues. The presence of these cells is usually associated with triple negative cancers. We have shown these are more often drug resistance to current chemotherapies than bulk tumour cells.
Contact
Cardiovascular Ageing and Regeneration
How does cellular senescence contribute to age related cardiovascular disease and dysfunction? Ageing is the biggest risk factor for impaired cardiovascular health. We have shown that during ageing there is an accumulation of senescence. This contributes to myocardial remodelling and increased inflammation.
We work on compounds that eliminate senescence cells in an in vivo model. Results show improved cardiac function, enhanced regeneration and improved outcome to myocardial infarction. Current work is on the mechanism by which senescence contributes to myocardial remodelling. In particular cellular stresses caused by myocardial infarction and ischaemia reperfusion.
Contact
Ex-Situ Heart Perfusion
We are exploring Hypothermic Oxygenated Perfusion (HOP). A novel approach to keeping the donor heart viable after organ retrieval. Cardiac preservation has remained unchanged for 40 years. This limits best organ utilisation as well as seriously impacting on transplant logistics.
Using a device originated by collaborators in Sweden. We study preservation and post-reperfusion functioning in human hearts declined for transplant. This is in concert with other organ perfusion approaches in the Transplant Regenerative Medicine lab. We are at the forefront of this technology with colleagues in Lund, Melbourne and Edmonton. Other collaborations include: Manchester, Cambridge and Oxford.
Contact
Paediatric Immunology and HSCT
Our team investigates immunoreconstitution following haematopoietic stem cell transplant (HSCT). This is a therapy for patients diagnosed with primary immunodeficiency disorder.
We monitor long-term outcomes of transplantation for primary immunodeficiency disorders. These include Chronic Granulomatous Disease and Severe Combined Immunodeficiency (SCID). We investigate DNA repair disorders and their treatments. In particular we focus on DiGeorge Syndrome.
We are developing new methods for T-cell depletion in patients with primary immunodeficiency. We have established a treatment protocol for extracorporeal photopheresis in children with graft versus host disease. This work has led to mechanistic insights into the action of extracorporeal photopheresis.
We are exploring defibrotide as a treatment of non-VOD endothelial cell activation disorders following HSCT therapy.
Contact
Toxicology, Liver Biology and Disease
Ionic liquids are a diverse range of charged chemicals. They have low volatility and are often liquids at ambient temperatures. They are considered as an environmentally friendly replacement for existing volatile solvents. However, methylimidazolium ionic liquids are slow to break down in the environment. A Newcastle study detected 1-octyl-3-methylimidazolium (M8OI). An 8-carbon variant methylimidazolium ionic liquid - in soils close to a landfill site.
We are looking at the toxicity of the methylimidazolium ionic liquids in general. Since little is known about this issue. We are considering the potential for M8OI - or related ionic liquids - to trigger primary biliary cholangitis (PBC). An autoimmune liver disease thought to be triggered by an unknown agent(s) in the environment.
Contact
Diabetes Research - Autophagy
Our group aims to understand the functional impairment and survival of pancreatic beta-cells in type 2 diabetes. These beta-cells play a critical role in regulating blood glucose homeostasis. In particular, how autophagy, impacts on both beta-cell function and survival. We aim to identify target pathways for therapeutic intervention.
We are also exploring the complex interplay between nutrient sensing and autophagy. These areas are particularly relevant in type 2 diabetes. Illustrated by the loss of beta-cell function and increased cell death.
Contact
Regenerative Medicine for Diabetes
It has been a century since the discovery and clinical implementation of insulin therapy. Despite many improvements diabetes self-management remains challenging and burdensome. Pancreas and isolated islet cell transplantation can normalise blood glucose. Liberating people from the need for insulin injections.
Professor Shaw leads the UK Islet Transplant Consortium. Our group is working from bedside to bench and back again towards achievement of:
- Reproducible sustained insulin independence after a single transplant procedure
- Beta-cell replacement without lifelong immunosuppression medication
Multimodality approaches aim to minimise beta-cell exposure to oxygen limitation and other stresses. We focus on novel advanced cell and gene therapeutics. Exploring these to improve cell survival and prevent cell rejection.
Recent evidence shows maintained islet mass and residual beta-cell function in all forms of diabetes. This opens up the exciting potential for restoring endogenous beta-cell function. By inhibiting stress signalling pathways impairing beta-cell function.
Our group is creating a unique bio bank. Containing whole pancreas tissue and isolated primary pancreatic cells. Alongside conducting metabolic studies, novel biomarker collections and detailed MR pancreatic imaging. We are exploring the relationship and signalling between the exocrine and endocrine pancreas. This encompasses type 1, type 2, malnutrition-related, cystic fibrosis-related and pancreatitis-related diabetes. In addition to allo- and auto-islet recipients.
Our goal is to:
- Pioneer novel therapeutics restoring normal pancreatic insulin secretion without long-term immunosuppression
Contacts
Joint Development and Degeneration
Using a range of in vivo and in vitro models our aim is to understand joint development. As well as identify the molecular mechanisms that underpin joint destruction. For instance in diseases like osteoarthritis (OA).
The groups focus is on gene regulatory mechanisms that control joint cell phenotype. Particularly that of chondrocytes. We aim to improve chondrocytes in development and disease. We manipulate gene regulatory programmes, identified through mathematical modelling. Our main area of study is non-coding RNAs, especially microRNAs. Though we also investigate other epigenetic mechanisms.
Contact
iPSC Models for Disease and Drug Discovery
Our group generates models of human disease using patient specific iPSC. Primarily for diseases of epithelial organs such as cornea, retina, inner ear, lung, kidney and liver. We have active research programmes building 3D models of these. Ensuring that they reflect the cellular architecture and functionality. In these we model the toxicity and efficacy of xenobiotics and prospective therapies.
An example is an iPSC based model of the pain sensitivity condition, Erythromelalgia. Used to search for novel analgesics. It uses sensory neurons derived from patient specific iPSC lines. Those carrying a mutation in the SNC9A gene. It screens small molecule compound libraries. Identifying candidates that slow the excessive firing rate of the diseased neurons. To date 67 prospective compounds have been identified.
Contact
Organ Donation and Transplantation
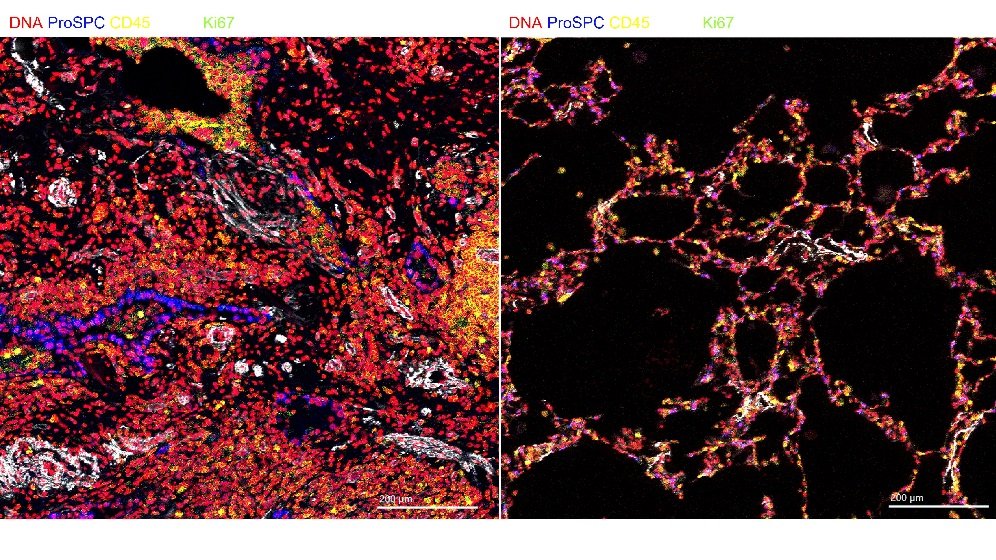
We evaluate new approaches to donor organ assessment, optimisation and subsequent transplantation. Aiming to increase the utilisation of donor organs whist maintaining excellent post-transplant outcomes.
We work on all solid organ transplants: heart, lungs, kidney, liver and pancreas. We are assessing a range of techniques to improve donor organ quality. These include ex-vivo perfusion technologies, gene therapy and cell therapy.
We are a multi-disciplinary group of clinical academics, NHS clinicians, scientists, engineers and computer scientists. We co-host the NIHR Blood and Transplant Research Unit in Organ Donation and Transplantation. A partnership with the University of Cambridge. (http://odt.btru.nihr.ac.uk/) We play a major role in the MRC Quality in Organ Donation programme. Hosting the lung, heart and pancreas platforms in Newcastle.
As part of this work we have major programmes in:
- Understanding cellular and molecular events occurring during ex-vivo lung perfusion
- Use of novel therapeutics to improve donated kidney function. Using normothermic perfusion before transplant
- Identification of biomarkers in machine perfusion of Liver. Using these to assess quality and predict outcomes
- Use of novel perfusion systems to preserve donor hearts. Assessing their function before transplantation
- Persufflation of pancreas using a newly engineered system. Partnership with Newcastle University spin-out ScubaTX
Contact


-and-AP2a-(green)996X560.jpg)