Ethics Forms and Processes
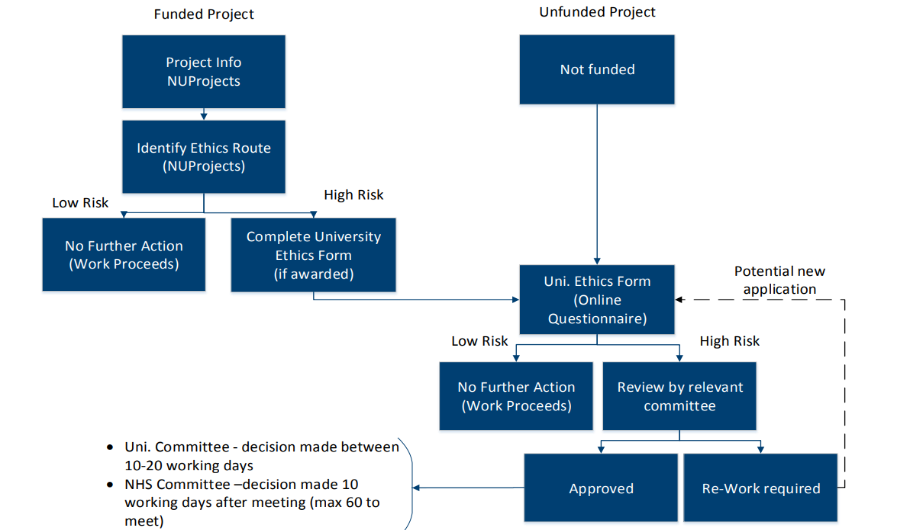
Staff and students at Newcastle University undertake thousands of (funded and unfunded) projects each year. Newcastle University’s ethics policy and procedure is designed so that ethics review is proportionate to the potential risk. The procedure is as follows:
Step 1: The principal investigator completes Newcastle University's Online Ethics Form. The form is made up of a series of questions, which aim to help the principal investigator identify whether the project is 'high risk' and requires further formal ethical review by a Research Ethics Committee.
Step 2: Once the form is completed and submitted, the principal investigator will receive a notification that either:
- based on the answers provided, the University is satisfied that the project meets the University's ethical expectations and grants the project ethical approval
- based on the answers provided, the project requires further review by a Research Ethics Committee before any research can begin
Where a principal investigator is applying for funding for a project, at the proposal stage (within NUProjects), the principal investigator is also asked to identify the ethics route that will be required for their project proposal (before the project is submitted and any funding is awarded).
Important note
Where a project requires further review, no work should begin until the project is granted ethical approval by the relevant ethics committee. The decision will be communicated directly from the relevant committee.
Ethics Process flowchart
Ethics Process flowchart (PDF:181kb)
Online ethics form
Newcastle University's online ethics form can be divided into two parts:
- preliminary questions, used to identify whether a project is considered high risk by Newcastle University
- additional information, used to acquire further information if the project is identified as requiring formal ethical review by a Newcastle University Ethics Committee
Please see sections below for further information.
Preliminary questions
The online ethics form will begin by asking for key details about the project (including, for example, the principal investigator, the title of the project, a short description of the project, details of any existing ethics etc.)
The form will then ask a series of preliminary questions to determine whether the project is considered high risk by Newcastle University, and therefore requires formal ethical review.
There are six sections:
- animals
- NHS, health and social care
- human participants
- data
- environment
- international
Additional information required for high risk projects
Where a project is considered as 'high risk' by Newcastle University (based on the answers to the preliminary questions on the online ethics form), the form will ask for additional information, including:
- the project outline and research methods
- data access, use and storage
- permissions required and/or obtained
- risk considerations and insurance
- supporting documentation, for example, participant information sheets, consent forms
Additionally, the form will ask further questions regarding the area of risk that was identified by the preliminary questions. For example, if the answers to the preliminary responses indicated that there was a potential risk to the environment, further information regarding this risk will be required.
It is recommended that a minimum of 30 minutes is set aside to complete the additional information section of the form. Should the form take longer than expected, it is possible to save the form by clicking the ‘Resume later’ button at the bottom of the webpage. To return to the form, click the 'Load unfinished survey' button at the bottom of the webpage.
Once a form has been submitted, amendments cannot be made. Should the information provided change, a new form will need to be submitted.
Outcomes
Depending on the answers provided to the preliminary questions, there are four potential outcomes:
- ethical approval required from an NHS/HSC REC (see Ethics Toolkit - NHS & Social Care)
- ethical approval required from the Animal Welfare Ethics Review Board (see Ethics Toolkit - Animals)
- ethical approval required from a Faculty Research Ethics Committee (see Newcastle University Ethics Approval Committees)
- no further ethical review required
Please see the sections below for further information.
Projects already granted ethical approval by an external ethics committee:
- the form will ask whether the project has already been granted ethical approval
- if so, the form will ask whether the Faculty Ethics Representative has agreed to accept the existing approval in lieu of a new application, see Ethics Toolkit - External Ethical Approval
- where the external ethics approval has been accepted, the form will not proceed to ask any further questions and the outcome will be: No further ethical review required
No further ethical review required
A copy of the form and any supporting documentation will be saved. The applicant will receive an email confirming the decision, which should be saved for their records.
Where a project is funded and awarded, the decision will be copied onto the relevant NUProjects record.
Ethical approval required from a University Ethics Committee
On submission, the form and any supporting documentation attached are sent directly to the relevant Research Ethics Committee (REC) for review. The applicant will receive an email confirming their application has been identified as requiring formal review by:
- Animal Welfare Ethics Review Board, or
- FMS REC
- SAgE REC
- HaSS REC
- School of Psychology REC
The project will then undergo formal review (please see Ethics Approval Committees for more details). The applicant will receive an email notifying them of the decision made by the committee, which should be saved for their records. Committees may:
- grant approval
- request further information
- request amendments
- reject the application
Research must not commence until the research has undergone ethical review and received approval from the relevant committee.
Where a project is funded and awarded, the decision (and any supporting documentation) will be added to the relevant NUProjects record.
Ethical approval required from NHS/HSC Research Ethics Committee
A copy of the form and any supporting documentation will be saved. The applicant will receive an email advising them that the project requires NHS/HSC Research Ethics Committee (REC) approval. For further information regarding how to apply for NHS/HSC REC approval, please see the Ethics Toolkit - NHS & Social Care.
Where a project is funded and awarded, the outcome of the NHS/HSC REC review will be recorded on the relevant NUProjects record.
Appealing a decision
Where a researcher wishes to appeal a decision reached by a Faculty Ethics Review Committee (FMS/HaSS/SAgE RECs), they should submit their appeal in writing to the Chair of the Committee, via the Faculty Ethics Coordinator. The Chair can reject, uphold or ask for an alternative review from within their own committee or another of the Faculty committees.
Following the Chair's decision, if the applicant is still dissatisfied, the matter can be referred for consideration by the Chair of the University Ethics Committee. Their decision is final. No research should start until the appeal has been resolved.
Amending approved projects
If you wish to make an amendment to a low-/high-risk project that has already been approved, please contact res.policy@ncl.ac.uk to clearly explain what it is that you require to change on your project. Based upon the information you provide, the amendment will either be categorised as low or high risk and will then follow these processes:
Low risk:
If the Research Policy, Intelligence and Ethics Team determine that this amendment is low risk they will amend the ethics survey that you originally submitted, to reference the amendment based upon the information that you have provided. You will be notified via email of this decision and will receive a pdf copy of the amended ethics survey relating to the project, for your records. No further actions are required following this process and you can implement the amendment to your project.
High risk:
If the Research Policy, Intelligence and Ethics Team determine that this amendment is high risk, they will notify you of this decision via email. You will be instructed to contact your Faculty Research Manager in order to inform them of the details of the amendment request and that it has been classified as high risk following the review by the team. Your amendment request will then be processed at faculty level, who will inform you of a decision directly.
